Chiral Center in Benzene Ring
Instead of using a rule-based numbering scheme to order neighbor atoms of a chiral center orientations are based on the order in which neighbors occur in the SMILES string. Substances containing the benzene ring are common in both animals and plants although they are more abundant in the latter.
Chiral Center Answers Ochempal
David Rawn in Organic Chemistry Study Guide 2015 166 The Williamson Ether synthesis.

. This produced a PdCCOF composite for catalysing Henry and reductive Heck reactions with high yield and excellent stereoselectivity. The chiral order of the ring closure bond is implied by the lexical order that the ring closure digit appears on the chiral atom. No-conflict chiral match where an H is present CCHClBr.
A carbanion is an anion in which carbon is trivalent forms three bonds and bears a formal negative charge in at least one significant resonance form. The Williamson ether synthesis is an S N 2 reaction in which an alkoxide ion is a nucleophile that displaces a halide ion from an alkyl halide to give an ether. To find a benzene ring which is not fused we write a SMARTS of 6 aromatic carbons in a ring where each atom is only in one.
Electrochemical Ring-Opening Dicarboxylation of Strained CarbonCarbon Single Bonds with CO 2. Reaction scope for catalytic reactions delivering THIQs. Li-Li Liao Zhe-Hao Wang Ke-Gong Cao Guo-Quan Sun Wei Zhang Chuan-Kun Ran Yiwen Li Li Chen Guang-Mei Cao and.
Indole is an aromatic heterocyclic organic compound with the formula C 8 H 7 NIt has a bicyclic structure consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. The chiral products 34 with 13-membered ring and 35 with 16-membered ring could both be obtained with moderate enantioselectivities. N in 5-sided aromatic ring nX2r5 Spiro-ring center X4R2r4r5r6r4r5r6r4r5r6r4r5r6r4r5r6rings size 4-6 N in 5-ring arom.
Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due to the presence of both hydrophilic CO 2 and hydrophobic alkyl regions in the same moleculeSuch molecules are termed amphiphilic Gk. 25 Recently Liu and co-authors condensed an axially chiral BINAPO linker with 135-benzene-triacetonitrile to afford a chiroptical CCOF which demonstrated the amplification of the chiroptical performance in. A conceptually simple example of phenylacetone organic synthesis is the Friedel-Crafts acylation of benzene with chloroacetone.
As an example benzene is written. The intermediate from the erythro tosylate is chiral but that from the threo tosylate is achiral note the plane of symmetry bisecting the three-membered ring. Of a stereogenic center and.
With a database of over 1 million transactions iChemical provides accurate price forecast for over 380000 chemicals and gets you chemicals from suppliers that best fit your sourcing budget. A newly discovered molecule contains a benzene ring. The generality of this asymmetric ring-closing strategy was further exhibited by its successful application to the synthesis of macrocyclic chiral compounds.
Various aromatics including benzene indole. R 3 CH B R 3 C. Formally a carbanion is the conjugate base of a carbon acid.
Fatty acids made up of ten or more carbon atoms are nearly insoluble in water. Where B stands for the base. Indole is widely distributed in the natural environment and can be produced by a variety of bacteriaAs an intercellular signal molecule indole regulates various aspects of bacterial.
Aqueous reduction of biomass-derived oxygenated compounds via magnetically induced catalysis was accomplished with core-shell FeCoNi nanoparticles encapsulated in carbon. Education for Ministry EfM is a unique four-year distance learning certificate program in theological education based upon small-group study and practice. Amphi both or amphipathic.
Multisubstituted biaryl skeleton by chiral phosphoric acid catalyzed desymmetrizationkinetic resolution sequence. Over 15000 chemical suppliers from all over the world are verified by iChemical according to their track record. Please read our Terms Conditions and Privacy Policy for information about.
This energetically efficient process opens the venue for the transformation of real biomass mixtures in a more sustainable way. 11-dimethoxy-1-phenylacetone - C11H14O3 synthesis structure density melting point boiling point. Plants can synthesize the benzene ring from carbon dioxide water and inorganic materials.
Assessment of various ligands showed that BOX ligands were the most effective ligand class and testing of other BOX-ligand variants led to further insights and improvements Replacement of the gem-dimethyl substituents with a spirocyclic cyclopentyl group L3 improved the enantioselectivity to 95 ee whereas use of tert-butyl substituents on the. Applications Phenyl acetone is used as an intermediate in the production of pesticides and anticoagulants. Next a pair of pi-electrons from the benzene ring bonds to C2 as the tosylate anion departs generating a phenonium intermediate in brackets.
This website uses cookies to help provide you with the best possible online experience. The fixed and rigid nature of the double bond creates the possibility of an additional chiral center and thus the potential. The reaction occurs with inversion of configuration at chiral centers and can be limited by possible competing.
Facile Synthesis of Diacids and Derivatization into Polyesters. In a molecule a center having four nonequivalent groups attached to it is known as chiral center Q. The carbanions formed from deprotonation of alkanes at an sp 3 carbon alkenes at an sp 2.

Finding Chirality Centers Youtube
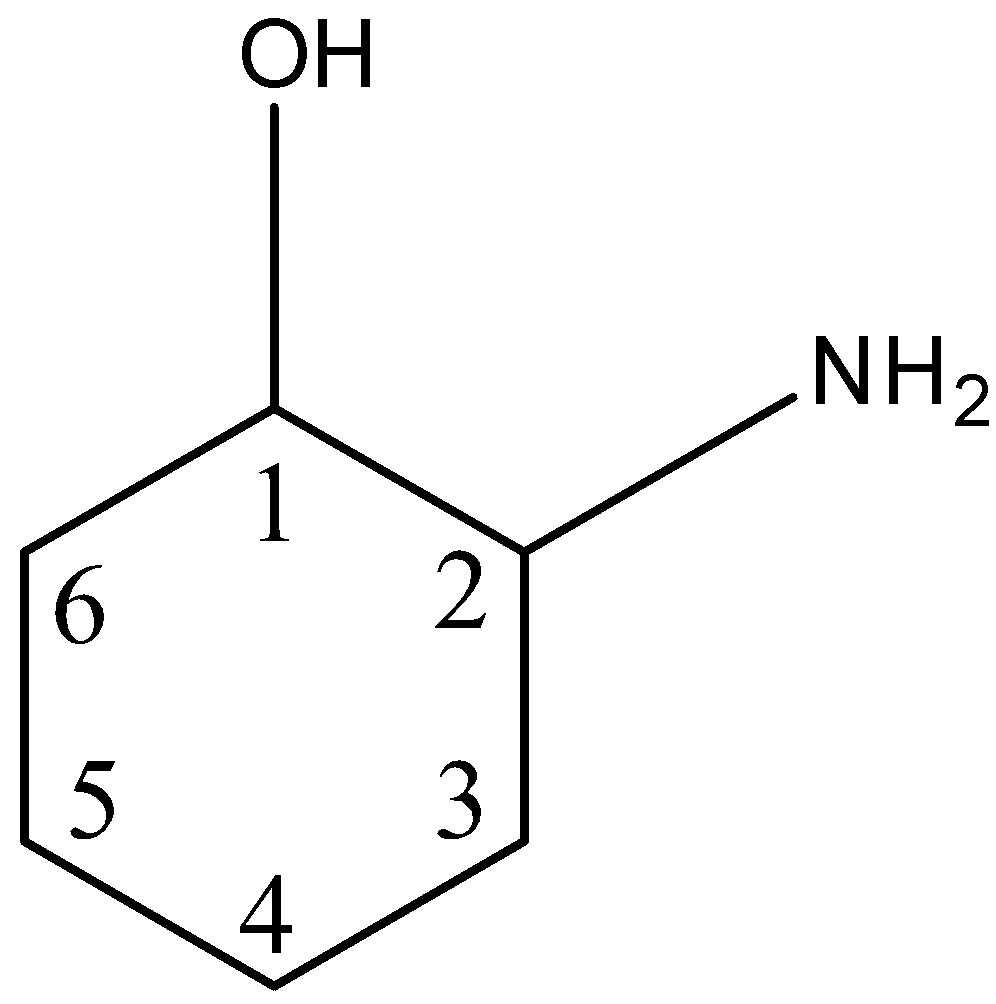
How Can I Find The Chiral Centers In The Ring Stru Class 12 Chemistry Cbse
How Can I Find The Chiral Centers In The Ring Stru Class 12 Chemistry Cbse
No comments for "Chiral Center in Benzene Ring"
Post a Comment